Developing Next-Generation Immunotherapies to Treat Cancer & Vaccines for Infectious Disease Prevention
Our proprietary biological adjuvant technology platform involves combining particulate antigen formulations with membrane-linked cytokine and immunostimulatory protein adjuvants. Our biological adjuvants are linked to the antigen source to provide an enhanced immune response and stimulate specific immune pathways to achieve the desired immune response.
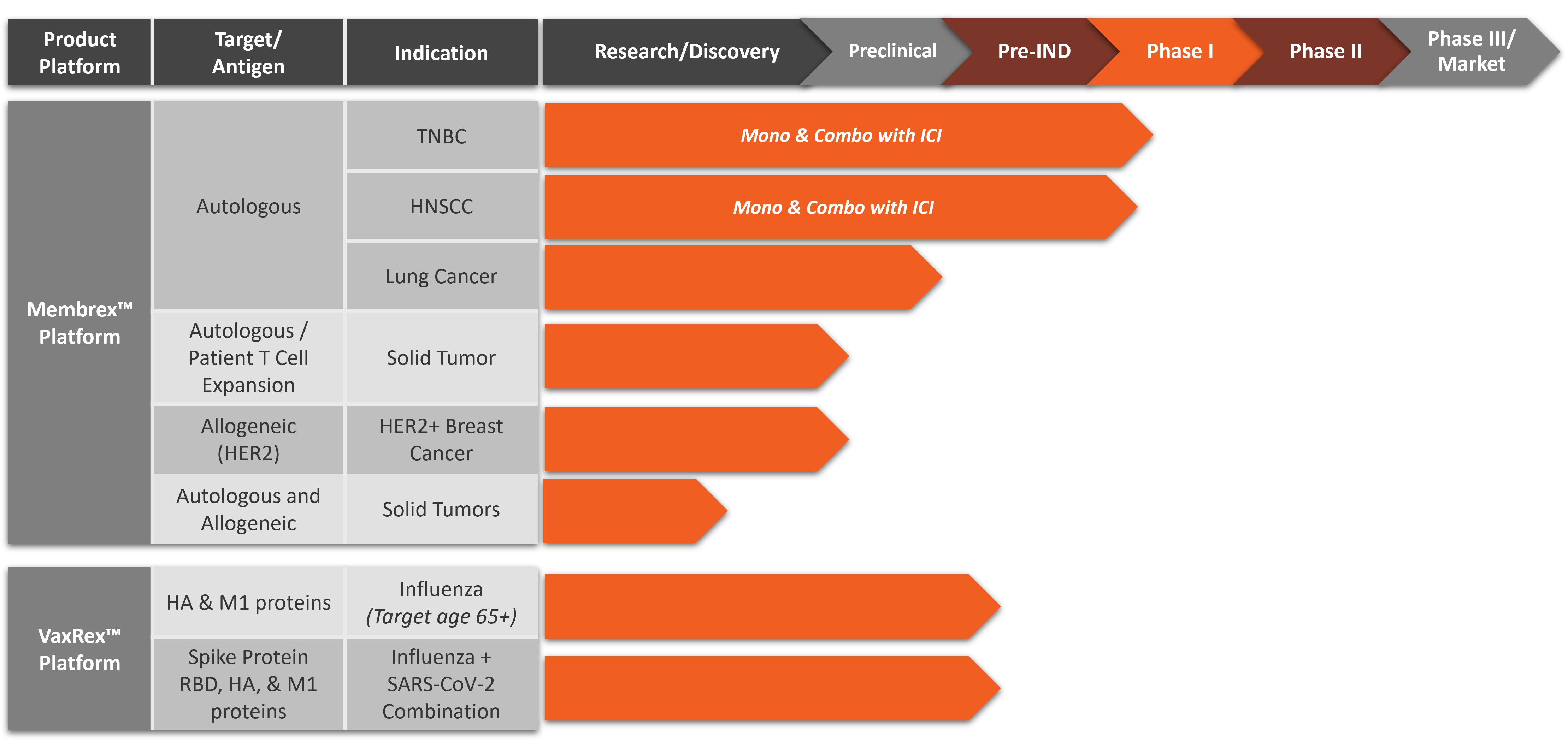
Membrex™ is an autologous immunotherapy platform that combines neoantigens and tumor-associated antigens from the patient’s tumor with our proprietary biological adjuvants. As our lead product platform, Membrex™ has remarkable potential to stimulate an immune response to immune checkpoint inhibitor (ICI) therapy-resistant and synergize with ICI therapy in preclinical cancer models. First-in-human Phase 1 clinical trials for advanced triple negative breast cancer and head and neck are expected to recruit patients in 2025.
Our VaxRex™ vaccine platform consists of antigen-expressing virus-like particles (VLPs) with our membrane-anchored biological adjuvants. These VLP-based vaccine products for infectious disease prevention are designed to elicit potent humoral and cellular immunity and provide superior protection from viral infection, especially for the elderly and immune-compromised individuals.
Metaclipse intends to commercialize its products through licensing agreements with major pharmaceutical companies after establishing safety and effectiveness.
Activating the Immune System to Eliminate Cancer
Membrex™ immunotherapy platform
Metaclipse Therapeutics is developing a novel personalized vaccine immunotherapy platform, Membrex™, as a monotherapy and as part of combination therapy for the treatment of multiple advanced cancers including triple negative breast cancer (TNBC), head and neck squamous cell carcinoma (HNSCC), non-small cell lung cancer (NSCLC) and HER2-positive breast cancer. First-in-human Phase 1 clinical trials for Membrex™ immunotherapy for triple negative breast cancer (TNBC) and head and neck squamous cell carcinoma (HNSCC) are expected to begin enrolling patients in 2025.
How Membrex™ immunotherapy is designed to work
Immune escape is one of major contributing factors for cancer progression. Further, cancer is highly heterogeneous and varies patient to patient. Membrex™, our cancer immunotherapy tailored to each patient and their specific tumor, consists of ‘membrane vesicles’ prepared from a patient’s tumor tissue modified using proven immunostimulatory proteins. After intradermal injection, Membrex™ delivers both immunostimulatory proteins and an array of patient-specific tumor-antigens simultaneously to the immune system resulting in suppression of cancer.
Membrex™ advantages over competitive immunotherapies
• Production does not require establishment of immortalized tumor cell lines, T cell cultures, or the use of viral vectors
• Includes all possible tumor antigens, addressing tumor heterogeneity
• Rapid, cost-effective manufacture and administration of the therapy to the patient within 3 weeks
• Particulate in nature, so optimal for uptake and processing by immune cells
• Physical linkage of biological adjuvants to tumor antigen source enhances immune response with a favorable safety profile
• Works in combination with immune checkpoint inhibitors (ICI) and enhances response rates for ICI-resistant cancers
Developing Membrane-Anchored Biological Adjuvants for Vaccines Against Infectious Diseases
VaxRex™ vaccine platform
We are developing a vaccine platform, VaxRex™, for infectious disease prevention consisting of ‘virus-like particles’ (VLP) modified using our proven biological adjuvants. After administration, VaxRex™ delivers both biological adjuvants and virus-specific antigens simultaneously in a particulate form to the immune system. VaxRex™ induced a robust virus-specific immune response in both young and aged animals. Metaclipse anticipates VaxRex™ will reduce infection rate and disease severity, especially for the elderly and immunocompromised individuals.
VaxRex™ advantages
• Broad applicability, including vaccines for enveloped viruses such as influenza, SARS-CoV-2, and HIV
• Rapid manufacture of the vaccine with the ability to achieve much higher surface concentrations of membrane-anchored biological adjuvants in comparison to gene transfer
• Easily degrade in the system, suggesting they do not accumulate in the body upon repeated vaccinations, avoiding chronic side effects
• Physical linkage of membrane-bound adjuvants with antigens yields potent immune response and favorable safety profile
Pipeline
News
January 2025 − Metaclipse Therapeutics is selected to participate in Texas Medical Center Innovation (TMCi)’s Accelerator for Cancer Therapeutics (ACT). Dr. Michael Coleman, Senior VP of Business Strategy, and Mr. Shaker J.C. Reddy, President have been accepted into the 2025 TMCi ACT cohort. The nine-month program, which kicked off in January, is funded by the Cancer Prevention Research Institute of Texas (CPRIT). Participants will have access to a curated mentor network, grant writing support, computational chemistry resources, and the dedicated Entrepreneur-in-Residence program. These resources are designed to equip participants with the strategic insights needed to secure investments, develop partnerships and advance the commercialization of cancer therapeutics in Texas. 2025 TMCi Accelerator for Cancer Therapeutics
November 2024 − Metaclipse Therapeutics secures $6M Cancer Prevention Research Institute of Texas (CPRIT) grant to advance head and neck cancer. Metaclipse Therapeutics has received a $6M grant from the Cancer Prevention Research Institute of Texas (CPRIT), as part of their Awards for Product Development Research announced following the CPRIT Oversight Committee Meeting on November 20, 2024. The funding will support the Phase 1 development of Membrex™ personalized immunotherapy in combination with an immune checkpoint inhibitor in recurrent head and neck cancer. CPRIT Funding Announcement
August 2023 − Metaclipse Therapeutics receives FDA clearance of its 2nd IND application for Membrex™ in head and neck cancer. Metaclipse received clearance from the U.S. Food and Drug Administration (FDA) for its 2nd Investigational New Drug (IND) application to initiate a Phase 1 clinical study of its personalized immunotherapy platform, Membrex™, in combination with an approved immune checkpoint inhibitor (ICI) for the treatment of recurrent and advanced metastatic head and neck squamous cell carcinoma (HNSCC) on August 23, 2023.
Membrex™ and VaxRex™ are investigational therapies and have not been approved for any indication by the Food and Drug Administration (FDA) or any other regulatory agency. The safety and efficacy of these therapies have not been determined.


